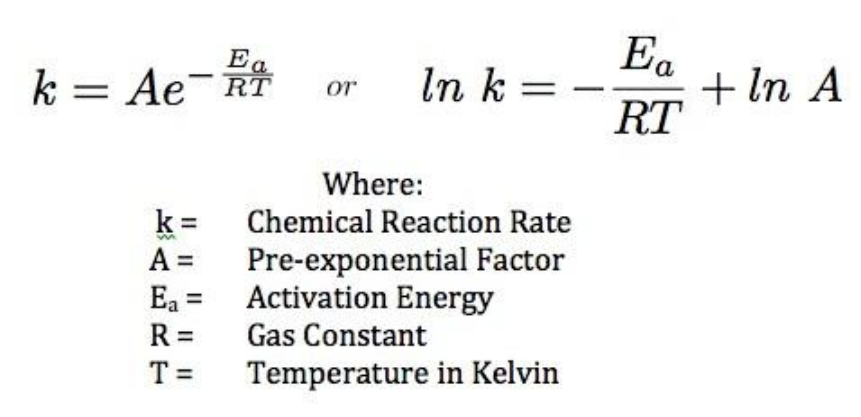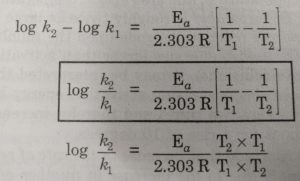Calculate the activation energy, Ea, in kilojoules per mole for a reaction at 65.0 C that has a rate constant of 0.288 s?1 and a frequency factor of 7.56 1011 s?1. | Homework.Study.com
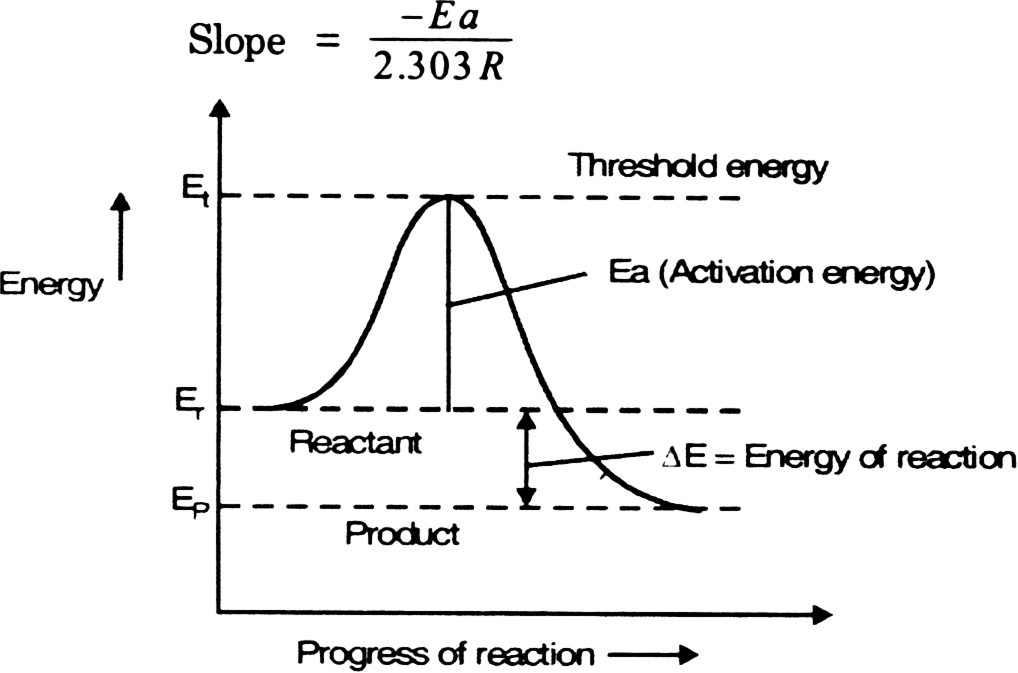
Draw a graph which is used to calculate the activation energy of a reaction. Give the appropriate expressions used to calculate the activation energy graphically. from Class 12 ISC Previous Year Board

Welcome to Chem Zipper.com......: The energy of activation for a reaction is 100 kJ mol^-1. Presence of a catalyst lowers the energy of activation by 75%. What will be effect on rate

Apparent activation energy calculation with Ph 2 CO mixed with C 3 H 8... | Download Scientific Diagram

Calculate Activation Energy for a Reaction of Which Rate Constant Becomes Four Times When Temperature Changes from 30 °C to 50 °C - Chemistry | Shaalaa.com

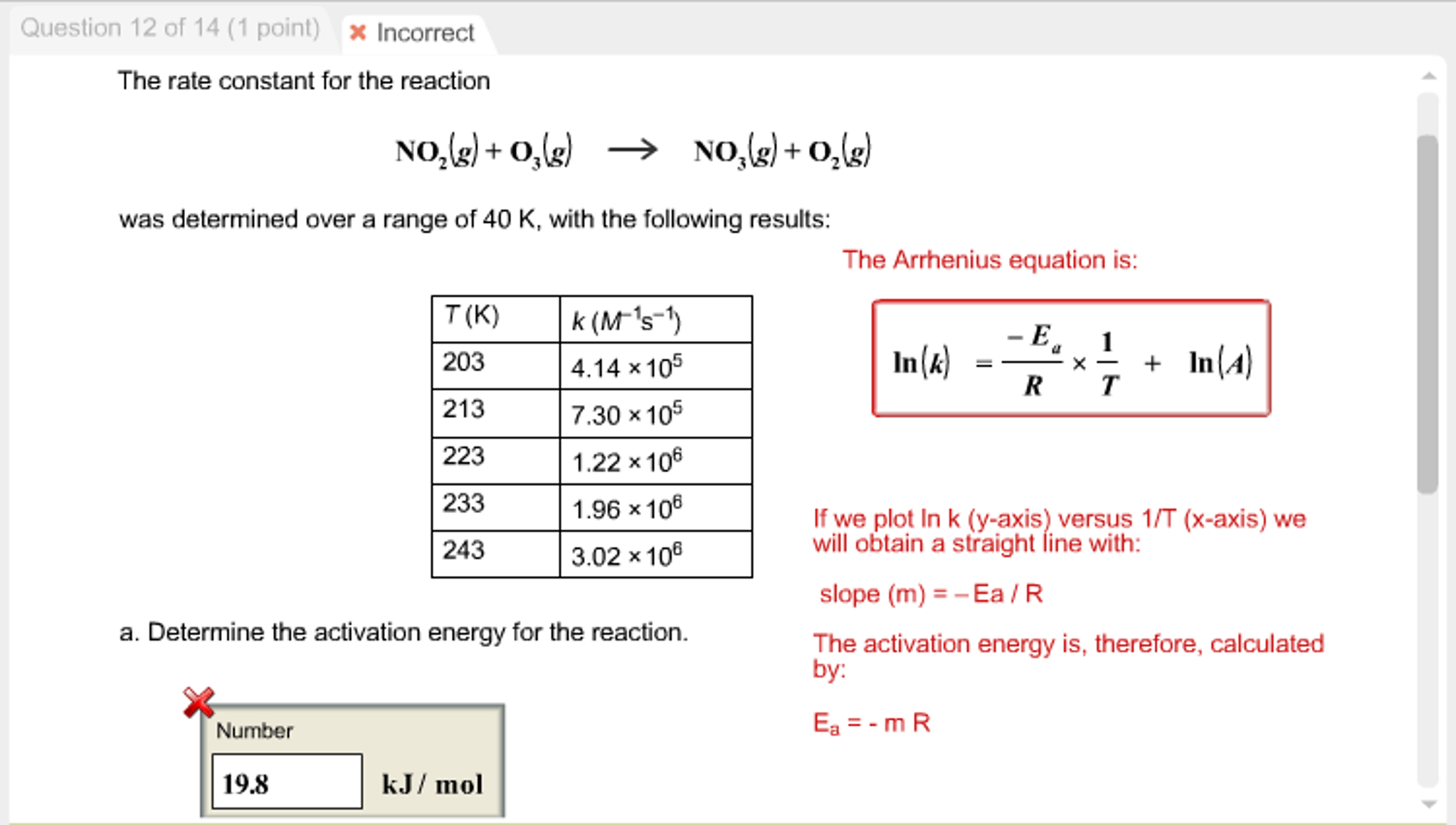
Title: Lesson 6 Activation Energy Learning Objectives: – Understand the term activation energy – Calculate activation energy from experimental data. - ppt download